Sulfur, Part I
A short discussion on a food preservative
I was giving a class on dehydrators at the Sustainable Preparedness Expo in Spokane last fall when during the Q & A time, the topic turned to the use of sulfur for fruit. To an observer, it would appear that there was a divide in the group of people who dehydrate into those who use sulfur and those who would never use sulfur at all—what is sulfur or sulfur dioxide? What does it do for my dehydrated food?
If you squint really hard, you can almost see us in this video
When I give a class, I always do my best to present the information on dehydrating, why you would do it, and how it works. Inevitably, the topic wanders into best practices for storage methods. By design I put the sales pitch for the Excalibur dehydrator dead last because I want to make sure that people have their questions answered about what the process is and how it works before we start talking about the best product for the money. I answered the question about sulfur that day by saying that I knew that it was there to preserve the color—and that was the extent of my knowledge on that. But, how does it do that? Does it have any other purpose? Are there any downsides to sulfur?
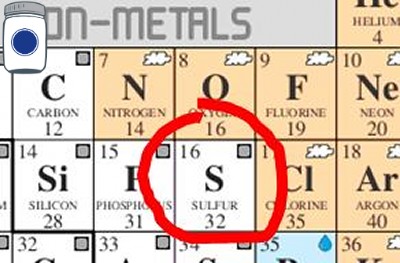
I found a mention of the 16th element on the periodic table of elements in a homesteading book, “The sick room in all cases and preferably every room in the house, in case of small pox, diphtheria, typhoid and other virulent diseases, should be thoroughly disinfected by fumigation. This may be accomplished by formaldehyde gas or by the fumes of burning sulphur” (Padgett, p. 104). Before we discuss whether or not burnt sulphur smoke will cleanse anything, let us clear the air on the spelling, is it “sulphur” or “sulfur?” It depends on who you ask, the British spelling (i.e. centre, colour, favour, fibre, etc.) is the former and the American spelling (i.e. center, color, favor, fiber, etc.) is the later—so I will use the American spelling since most of my sources used that variant.
Sulfur, like most chemical elements is a yellowish-solid at room temperature, which is likely a good thing because it has much to do with all kinds of finished goods from dehydrated food to high fructose corn syrup and other “products and processes that include fabric dye, ink, water purification, wood preservation, and weed killers” (Ettlinger, p. 34). Other than its more industrious applications, sulfur is also used as an antimicrobial food preservative. “If everyone grew and cooked their own food, and everything we ate could be guaranteed fresh and safe, there would be no need for food additives” (Joachim & Schloss, p. 247).
Your alternative to eating foods containing preservatives is to visit the farmers’ market every day for fresh meat and produce. Also make your own cream, preserves, pickles, cheese, wine, potato chips, cereals, and olive oil, being sure to consume them before they go bad.
And welcome to the eighteenth century (Wolke, p. 375)
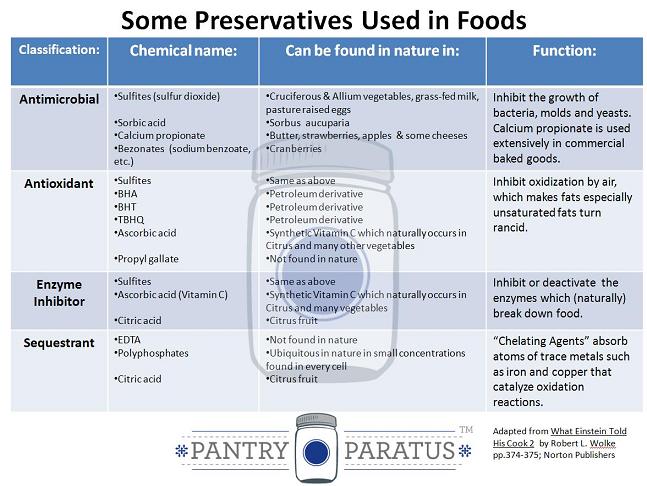
To that end, man has chiefly sought out to live better through chemistry with food additives. Lest the discussion slide down directly into a big corporate bashing session, people have been using salt, smoke, oil, fermentation and sugar to preserve food for millennia (Joachim & Schloss, p. 247). Whenever I present on dehydrating at an expo, I am covering a two pronged approach to denying bad bacteria (or any living thing) the ability to decompose your stored food: removing water and removing oxygen (further bonus points go to controlling temperature, excluding vermin and eliminating light). Since consumers do not buy very many food products that are vacuum sealed in opaque packages with oxygen absorbers (food marketing agencies say that we eat with our eyes), we have to ingest a myriad of chemicals to postpone the decomposition of our food. “Enter preservatives: chemicals added to prepared foods to extend their shelf lives—and the lives of us who eat them” (Wolke, p. 374). Sulfur happens to be a natural variety of such a preservative.
Here is a chart of some preservatives used in food (sulfur is listed under antimicrobial and antioxidant as sulfites):
So is sulfur bad for you? Not at all. “As the remarkable properties of vitamins have revealed themselves to investigators, so too have those of the various minerals in our food and water. The seven macrominerals—calcium, chloride, magnesium, phosphorus, potassium, sodium and sulfur—now share the spotlight with a longer list of trace minerals” (Fallon & Enig, p. 40).” Actually Sally Fallon Morell talks a lot about sulfur-containing proteins as assisting in a host of cellular functions to include maintaining the cellular wall and protecting the body from infection, blocking harmful effects of radiation and pollution as well as slowing down the aging process (pp. 43, & 436). As you see, sulfur can not only slow down the aging process of food, but of you as well!

(No, that is not Chaya)
Sulfur indeed is an interesting mineral “known biblically as brimstone, is perfectly odorless, but many of its compounds are evil smelling. Sulfur dioxide is the smell of burning sulfur” (Wolke, p. 28). But how does sulfur work to do all of this? What does sulfur have to do with dehydrated food? We will get into that in Part II in the next blog. I will give you a hint, it has to do with the third most abundant element in our universe and ever present in living things (H2O).
Wilson
Pro Deo et Patria
Works Cited:
Ettlinger, S. (2007). Twinkie, deconstructed, my journey to discover how the ingredients found in processed foods are grown, mined (yes, mined), and manipulated into what America eats. (First printing, March 2007 ed., Vol. 1). London: Hudson st Pr.
Fallon, S., & Enig, M. (2001). Nourishing traditions. (p. 40). Washington DC: Newtrends.
Joachim, D., & Schloss, A. (2008). The science of good food. (p. 239). Toronto: Robert Rose.
Padgett, C. (2001). Keeping hearth & home in old Ohio. (p. 109). Birmingham: Menasha Ridge Press.
Wolke, R. (2005). What Einstein told his cook 2: Further adventures in kitchen science. (p. 375). New York: W. W. Norton & Company.
Photo Credits:
Sulfur: Periodic Table of elements taken from the public domain: http://www.wpclipart.com/education/supplies/periodic_table_of_the_elements.png.html
Sulfur Dioxide: photo credit: <a href=”http://www.flickr.com/photos/lipson/524489441/”>joe.lipson</a> via <a href=”http://photopin.com”>photopin</a> <a href=”http://creativecommons.org/licenses/by-nd/2.0/”>cc</a>
Chemistry Equipment photo credit: <a href=”http://www.flickr.com/photos/fdctsevilla/4623395359/”>El Bibliomata</a> via <a href=”http://photopin.com”>photopin</a> <a href=”http://creativecommons.org/licenses/by/2.0/”>cc</a>
Antimicrobial collage:
photo credit: <a href=”http://www.flickr.com/photos/nathanreading/6795865300/”>Nathan Reading</a> via <a href=”http://photopin.com”>photopin</a> <a href=”http://creativecommons.org/licenses/by/2.0/”>cc</a>
photo credit: <a href=”http://www.flickr.com/photos/adonofrio/4478011500/”>adonofrio</a> via <a href=”http://photopin.com”>photopin</a> <a href=”http://creativecommons.org/licenses/by/2.0/”>cc</a>
photo credit: <a href=”http://www.flickr.com/photos/66126733@N04/6287939381/”>Rising Damp (busy, silent)</a> via <a href=”http://photopin.com”>photopin</a> <a href=”http://creativecommons.org/licenses/by/2.0/”>cc</a>
Oxygen: Periodic Table of elements taken from the public domain: http://www.wpclipart.com/education/supplies/periodic_table_of_the_elements.png.html
Further Reading:
CDC article on “Sulphur Mustard” otherwise known as that horrendous substance, “Mustard Gas.” http://emergency.cdc.gov/agent/sulfurmustard/ This correctly outlawed chemical warfare toxin comes from the mustard’s seed:
A member of the brassica family, related to broccoli and cabbage, mustard is valued for its seed, which contains a compound called sinigrin. During grinding, enzymatic action liberates the pungent principle from the sugar molecule to which it is attached. Sulphur [sic] compounds and oils are also released. These compounds have a penetrating odor and an irritating effect on the skin and mucous membranes. (Morell, Enig, p. 104).
Sulfur compounds are also what accelerate both the decomposition of fish flesh making it smell putrid or the pleasant aromas from garlic or onions. “Much of the aromatics in roasted coffee beans come from mercaptans, which are sulfur compounds” (Joachim & Schloss, p. 239).
BHA cause for concern; an article citing research suggesting a link to cancer: http://ntp.niehs.nih.gov/ntp/roc/twelfth/profiles/ButylatedHydroxyanisole.pdf
Great article from Medopedia.com on “Petroleum, it’s what’s for breakfast” http://www.medpedia.com/news_analysis/98-Small-Bites/entries/79619-Petroleum-Itrsquos-Whatrsquos-For-Breakfast
Proviso:
Nothing in this blog constitutes medical advice. You should consult your own physician before making any dietary changes. Statements in this blog may or may not be congruent with current USDA or FDA guidance.